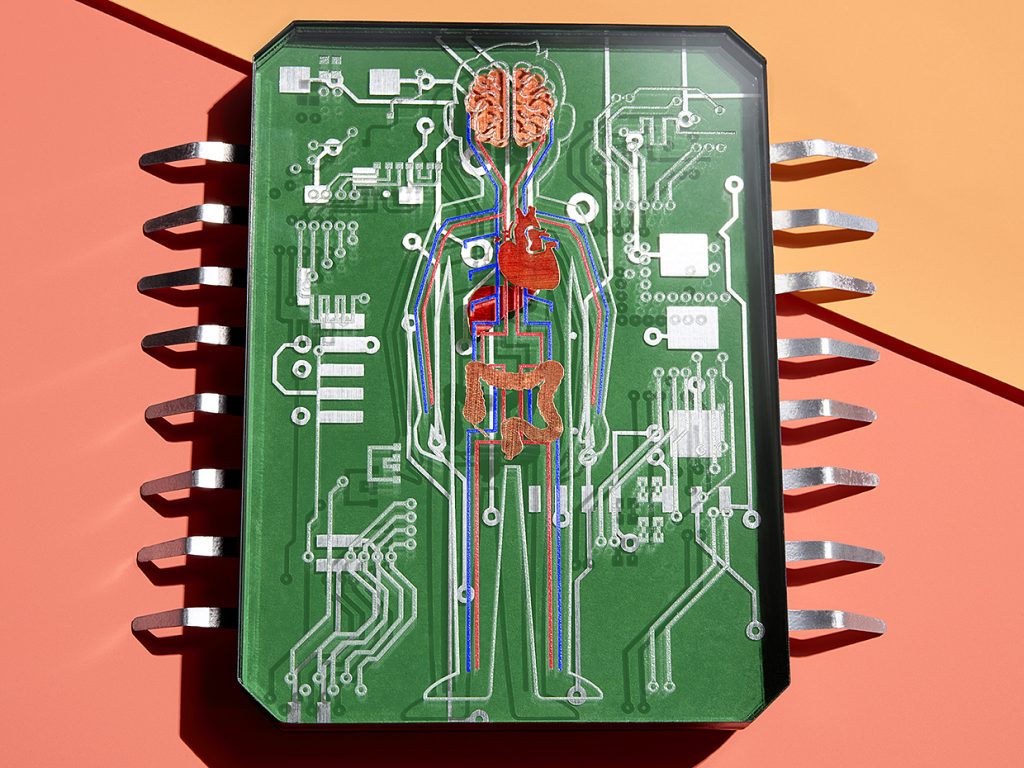
You’ve fallen ill, but neither you nor your doctor know which treatment will work. Which would you rather do—try five different drugs, one at a time, until you find one that treats your illness without serious side effects, or take one drug that’s guaranteed to work? You’d opt for that one drug, of course.
Right now, though, there’s no way to know for certain that a particular medication will work in your particular case. But someday, before you ever take that drug, it could be tested on a version of you small enough to fit in your pocket.
These miniaturized copies of you would be enabled by improvements to technologies that are currently in development in labs around the world. These organ-on-a-chip devices—usually made on substrates of plastic or rubber, not silicon—contain living cells. These cells are organized to form a 3D bit of artificially grown tissue, often called an organoid, that operates like a human organ but on a scale of cubic millimeters. A liver organoid might be functional enough to metabolize the painkiller paracetamol. A lung organoid could simulate breathing.
An organoid on its own is useful, but in your body, no single organ works in isolation. Your organs are in constant communication. Your nervous system sends commands to the rest of the organs to modulate their behaviors; your heart pumps blood to other organs to deliver oxygen and nutrients; the pancreas produces insulin that tells everything else how much glucose to take in. And we can’t know for sure the real therapeutic value of a new drug or its side effects unless we can test it in a system more complex than just one organ. So researchers, including my group at Harvard Medical School and at Brigham and Women’s Hospital, in Boston, have been developing chip-based systems with multiple organoids—systems with a miniature heart, a diminutive liver, even a basic brain. Many of these are 3D printed and all are connected by a circulatory system of microfluidic pumps and channels.
Today scientists are using these systems to figure out how drugs work inside the body, find new therapies, and understand how cancer spreads, among many other things. And one day researchers will be able to use your own cells in these systems to predict how drugs will work in you and how to defeat your cancer. That’s important because the same drug can have a different effect—or side effect—on you than it would on somebody else, even someone in your own family. There are indeed still many mysteries about how relatively common drugs work, and a number of blockbuster drugs help only a fraction of those to whom they are prescribed.
Getting to the point where we can produce person-specific systems that can solve these mysteries will require advances in stem-cell research as well as in biofabrication, but this most personal of approaches to personalized medicine is definitely in sight.
solve these mysteries will require advances in stem-cell research as well as in biofabrication, but this most personal of approaches to personalized medicine is definitely in sight.

Liver Lobules: The hexagonal structure of liver tissue can be mimicked by the photolithographic patterning of liver cells. Image: Y. Shrike Zhang/Zhang La
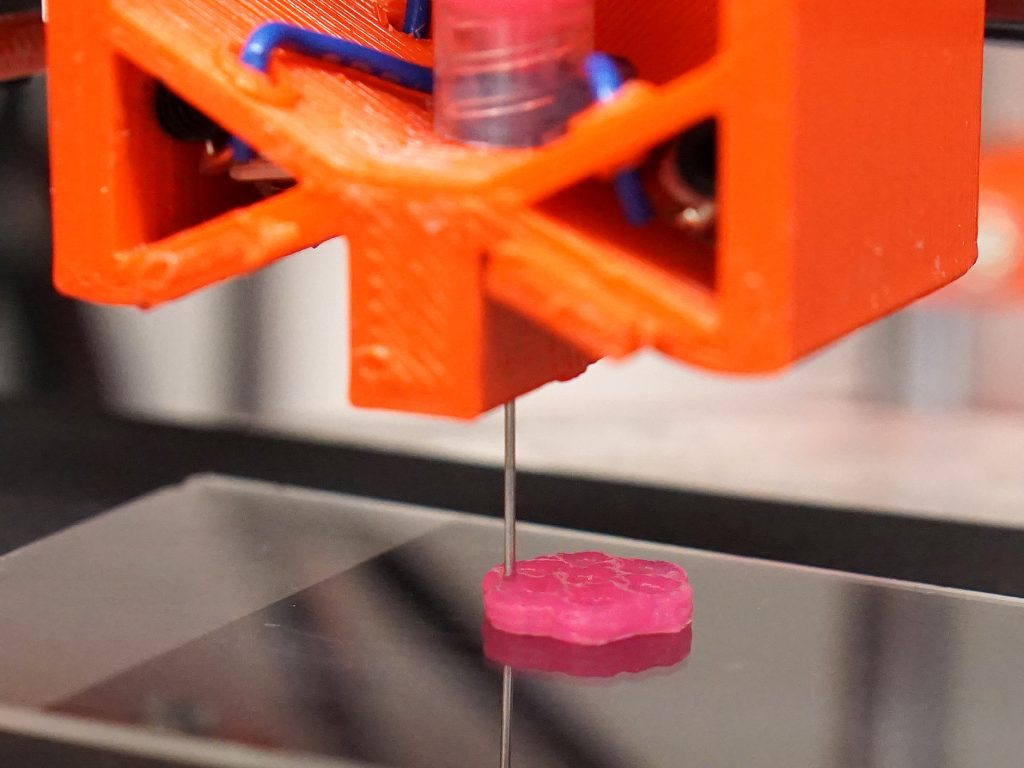
Get Organized: Organoids are cells structured to produce some of the physiology of an actual organ. Photo: Y. Shrike Zhang/Zhang Lab
The first organ-on-a-chip systems were created in the early 2000s to study the interaction of drugs. Called micro cell culture analogs (microCCAs), they consisted of living cells either on a surface or embedded in a 3D matrix of hydrogel and connected to fluid flow by channels with dimensions measured in hundreds of micrometers.
Since then the technology has grown in both scope and complexity. Today’s devices are made of multiple types of cells, and they now closely mimic some of the internal microstructure and function of the organs they represent. Although the terminology of what microCCAs have become is somewhat fluid, we call them microphysiological systems.
Getting the right type of cell to survive in the right place requires precise control of many variables at multiple scales. In your body, biomolecules that make up functioning tissue at atomic scales assemble themselves into nanoscale macromolecules. Those macromolecules then create micrometer-scale building blocks and then tissues and organs. When your body builds a liver, for example, it combines several types of cells. The main liver cells, called hepatocytes, are assembled with hepatic stellate cells (which lie dormant unless the liver is damaged) and Kupffer cells, which are immune system cells that live in the liver. Together these cells form hexagonal units of tissue called lobules. Lobules contain ducts that secrete bile for digestion, as well as blood vessels that deliver oxygen, remove CO2, and carry substances absorbed by the gastrointestinal system for the liver to metabolize. The lobules are closely packed together to produce the macroscale structure of your liver.
Replicating such levels of tissue complexity and their associated biological functions on a chip is not a trivial exercise, but it can be done. To reproduce the liver lobule’s hexagonal microstructure, researchers adapted the chipmaking technique photolithography. The liver cells can be encapsulated in a biocompatible photosensitive hydrogel, a substance made of water-loving molecular chains that bind into a web when exposed to the right color of light. The cells are loaded into a specially designed milliliter-scale chamber, and a repeating few-hundred-micrometer-wide hexagonal pattern of light is cast onto them, setting the hydrogel and fixing the cells in place.
Three-dimensional bioprinting is another promising technique for turning a collection of cells into an organized organoid. This is similar to 3D printing, except that the final structure is living tissue. Fundamentally, you’re just extruding or otherwise printing a pattern of “bioink”—that is, cells in a photosensitive hydrogel precursor—and fixing the pattern in place by zapping it with light. But in reality, it’s more complex than that. Cells are sensitive, and they react—sometimes strongly—to mechanical forces, such as sheer stress. So the printer’s flow rate and other parameters must be carefully controlled.
The right mix of hydrogel is important. For example, together with scientists in Europe, we recently produced a 3-D bioprinted mini-brain organoid. Hoping to explore how a type of cancer cell invades the brain, we gave our mini-brain a tumor. The mix of ink was crucial. It had to be thick enough to give the organoid and its tumor a shape before it was fixed in place. And once it was fixed, the gel had to form pores big enough for the tumor cells to communicate with the ordinary brain-residing immune cells.
Using 3D bioprinting, photolithography, and a range of other techniques, research teams have created all sorts of organoids that reproduce certain functions of their counterparts in the human body—intestines that absorb nutrients, cancers that invade other tissue, heart muscle that contracts, even a lung that inhales cigarette smoke.
But the real value comes when you link organoids together. Michael Shuler’s group at Cornell University built a microphysiological system that contains three types of organoids—liver, bone marrow, and a tumor of the colon—on a single chip with closed circulation. Researchers used it to examine the metabolism of a decades-old anticancer drug called 5-fluorouracil (or 5-FU). When 5-FU is administered orally, its effects are limited because the amount that actually gets to the tumor has proved unpredictable. To solve this issue, researchers developed a more stable molecule that the body metabolizes to become the active drug. Tegafur is one of these prodrugs of 5-FU.
By itself, Tegafur isn’t toxic to the patient or the cancer. But when metabolized by an enzyme in the liver, it becomes effective against the cancer and remains active in the body much longer than oral 5-FU does. Using a liver–colon cancer–bone marrow system, researchers managed to reproduce the way the liver metabolizes the Tegafur. As expected, the Tegafur itself did not harm the colon cancer organoid, whereas after liver metabolism, the modified drug proved fatal to it. It was also pretty toxic to both the liver organoid and the bone marrow organoid. The experiment was one of the first to show how multiple-organoid systems can recapitulate human responses to drugs.
Linking multiple organoids together is tricky. Because these microphysiological systems are miniaturized, their operating parameters can be way off from those of human organs, which are thousands of times larger and heavier. You probably know that small mammals usually have faster heart rates and metabolisms than do large ones. As we try to re-create functional human systems on small artificial chips, we certainly do not want to create something that is made of human cells but metabolizes like a mouse.
So properly scaling down these systems is crucial. You have to solve two problems. One is the scaling of the organoids relative to their human counterparts. This concerns how you go about translating real patient dosages of drugs into the concentrations of those drugs tested on the chip. The other problem is scaling the flow rate of fluids among the organoids so that they reproduce how the drugs get distributed and metabolized in the human body.
Scaling has usually been done according to the relative sizes of the organs in question and the flow rates they experience. But more recently, a new scaling strategy has been developed that may deliver better results. This strategy proposes that microphysiological systems should be designed based on a combination of their purpose and how they actually achieve that purpose. For example, if an animal’s gut is known to absorb a set of drugs at a particular rate and its liver metabolizes those drugs at a certain rate, then a microphysiological system that includes a miniature gut and a miniature liver should be scaled to do its work at those same rates. Then when the system is used to explore the actions of new drugs, it will likely have been scaled correctly.
Another challenge is measuring what’s happening in a multiorganoid system. In daily life, patients may undergo a series of medical exams, from noninvasive tests using a stethoscope to invasive tests requiring surgical biopsies. For today’s organoids, testing isn’t just invasive; it’s usually destructive. Most analyses rely on staining the organoids with chemicals that bind to specific biomolecules—an irreversible process that means the system can’t be reused.
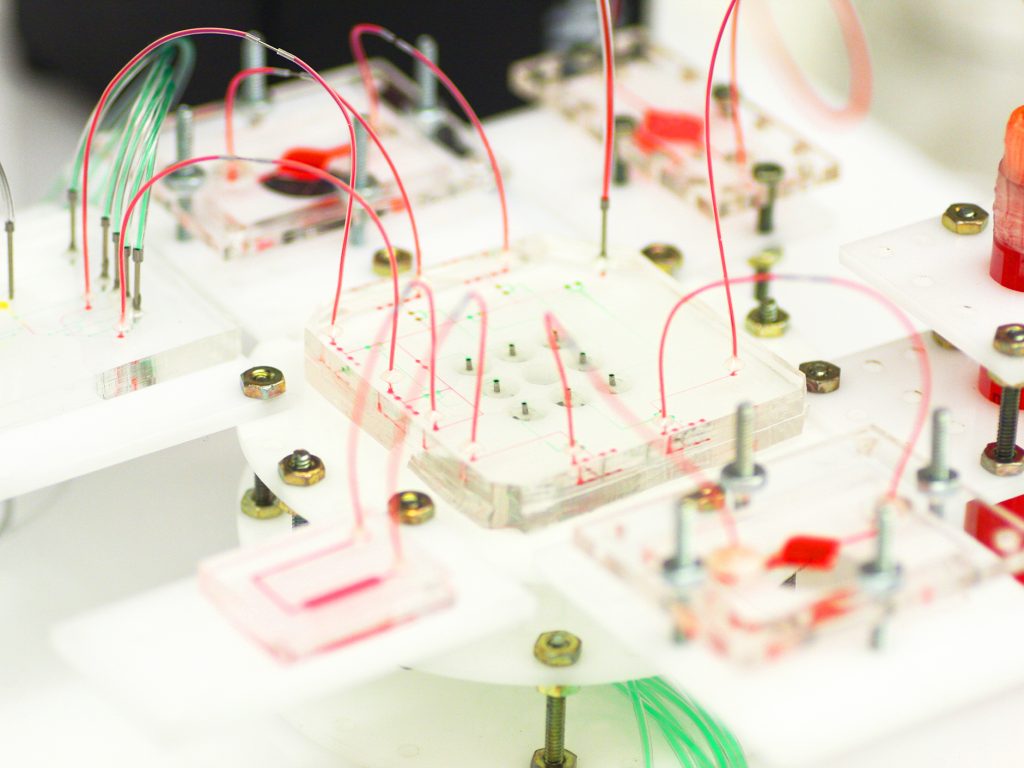
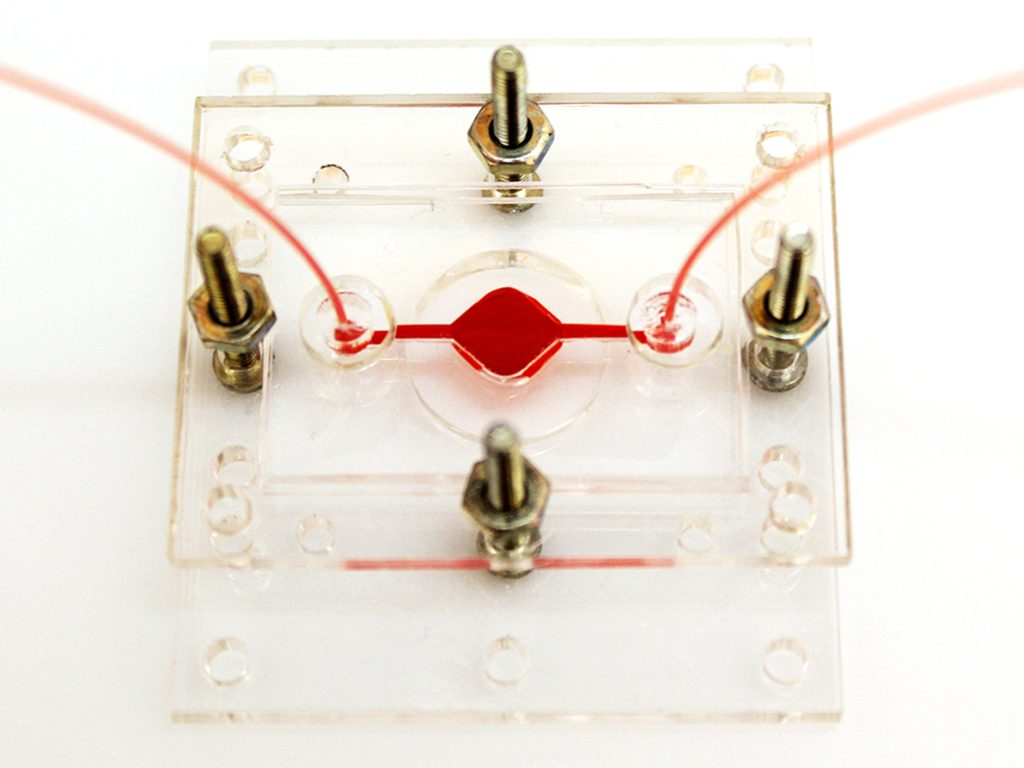
A Reconfigurable Microphysiological System
Pneumatic control lines [green] can reconfigure the flow of fluid [red] through a system that can contain multiple organoids. An electrochemical sensing chip [far left] can connect to the system to detect different biochemicals in the fluid.
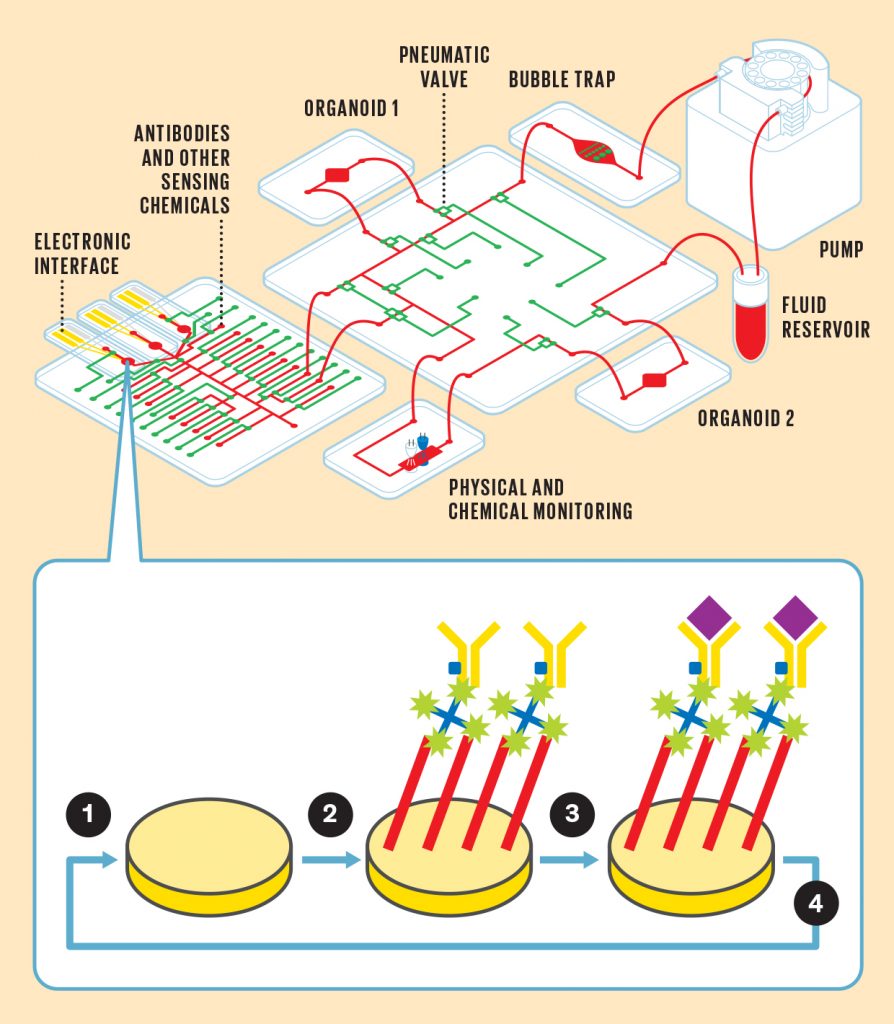
1. Electrodes sense the presence of a particular molecule as a change in resistance. But first the electrode must be loaded with the proper chemicals to enable that sensing.
2. A series of chemicals are affixed to the electrode, including an antibody [yellow] that is uniquely sensitive to the molecule you’re looking for.
3. The molecule [purple] binds to the antibody, causing a change in resistance in the electrode.
4. The electrode’s chemical coating can be removed and a new one reloaded in order to sense a different molecule.
Other analyses remove fluid from the system to measure biomolecules secreted into the cell culture medium. Although that’s similar to blood tests done in human patients, it’s not quite suited for the miniaturized organ-on-a-chip systems, because the latter typically require much more frequent monitoring than the human body does, and each measurement requires a comparatively large volume of fluid. Imagine the impracticality and discomfort of giving a patient 5 to 10 blood tests per day, and each time drawing 50 to 100 milliliters of blood. The average human body has only about 5 liters of blood, and you’d probably be dead after losing 2 liters.
A more rational approach for measuring organoid performance is to build miniaturized sensing units that are part of the same microfluidic circuit that connects the organoids. The sensors can be integrated into the microphysiological systems themselves. That way, a minimal volume of liquid is sampled, and multiple samples can be taken from what is now a reusable system.
To that end, my team and collaborators have built a reusable electrochemical sensing system that registers the presence of target molecules by a change in resistance. Electrodes in this microfluidic chip can be coated with a series of chemicals, including antibodies that are designed to bind only to the molecules you want to measure. When those target molecules bind to the electrode, the electrode’s resistance increases in a way that indicates the chemical’s concentration.
What’s unique to our system is that the same electrode can be reused for different targets while the experiment is still running. A network of automated microfluidic channels and pneumatic valves supplies the mixtures needed to remove the old coating from the electrode and apply a new coating.
How will we move from today’s research-grade multiorganoid chips to a chip-scale duplicate of a person that can be used to test drug effectiveness and scan for potential side effects? One challenge is that the chip’s organoids must be made from the patient’s own cells. For a chip that includes a cancer organoid, that means performing a biopsy on the tumor, something that’s often done in the course of diagnosis anyway. But for other types of organoids, such as the liver or lungs, it means turning one type of cell into another.
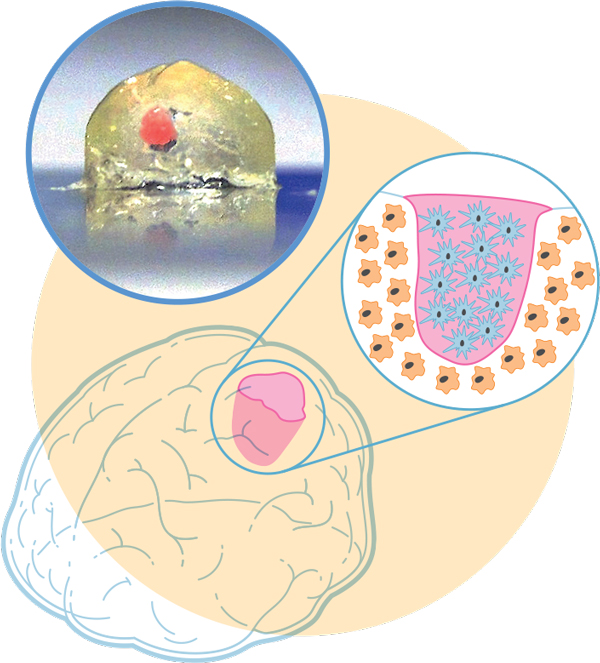
Illustration: James Provost; Photo: Marcel Heinrich
Mini-brain, Meet Mini-tumor: In order to study a type of brain cancer, we 3D-bioprinted a millimeter-scale brain using immune-system cells called macrophages. In a cavity of the mini-brain, we bioprinted cells from a glioblastoma tumor—the most aggressive cancer that initiates in the brain. The two cell types interacted, with the tumor recruiting the macrophages to help it grow and spread.
The body has a way to do just that. Stem cells are cells that have the potential to become any type of cell—neuron, bone, liver, skin, anything. When researchers first began studying them in the 1980s and 1990s, stems cells were difficult to procure, requiring the manipulation of embryos. A little over a decade ago, however, Shinya Yamanaka and researchers in his lab at Kyoto University discovered a much easier way. (And he won a Nobel Prize for it.) By manipulating a set of four genes, Yamanaka turned an ordinary adult skin cell into the equivalent of a stem cell. These repurposed cells, called induced pluripotent stem cells, can be manipulated to make any type of tissue you want. Most important for future diagnostic organ-chips is that the organoids created from these cells are a genetic copy of whoever supplied the original cells. Right now, this process is time-consuming, taking weeks that a patient might not have, but it is improving.
Organoid fabrication methods will have to improve as well. No one approach is likely to suit every need: 3D-bioprinting can produce intricate structures, but it’s typically a low-throughput technology compared with photolithography. Other techniques such as molecular self-assembly—where the chemical nature of polymers and other molecules causes them to form nanoscale or microscale structures without outside guidance—may need to play a part. As is often the case, a combination of technologies may be required.
Despite the gulf between what we can do now and what’s wanted, personalized microphysiological systems are worth the effort. We are all biologically individuals. A painkiller that works well for you may not work for even your closest family member, and that in turn may mean a danger of overdose. The ability to accurately understand our uniqueness, despite the extreme complexity of human physiology, could make all the difference.
This article appears in the April 2019 print issue as “A Medical Mini-Me.
source : www.spectrum.ieee.org





















































