In an exciting new bioRxiv* preprint research paper, Chinese researchers describe the development of membrane nanoparticles from ACE2-rich cells with a potent capacity to block the adherence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The relentless coronavirus disease (COVID-19) pandemic quickly prompted the pursuit of novel effective therapeutic agents against the highly virulent SARS-CoV-2 virus. One of the prime targets for researchers is the angiotensin-converting enzyme 2 (ACE2), the primary receptor for SARS-CoV-2 spike glycoprotein (S1 subunit), which mediates viral entry into host cells.
It is already known that ACE2 is pervasive in lung alveolar epithelial cells, renal tubular epithelium, and enterocytes of the small intestine, as demonstrated by large-scale immunohistochemistry, transcriptomics, and proteomics analysis.
Due to the natural positioning of ACE2 on the cell membrane, and considering how membrane with active ingredients can be used to expand our antiviral arsenal, the researchers from the Third Military Medical University in Chongqing (China) attempted to apply the membrane of human cells abundant with ACE2 to cope with SARS-CoV-2.
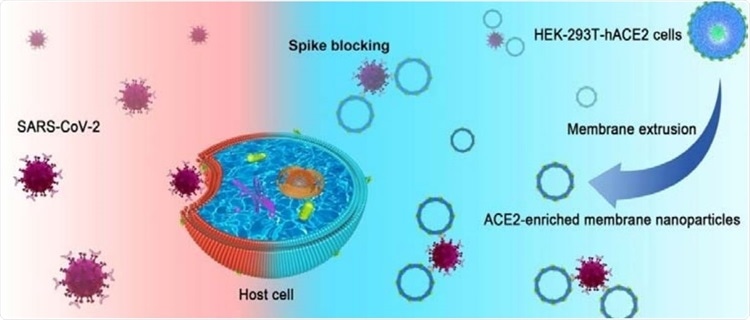
Developing cell membrane-based nanoparticles
In this study, cell membrane-based nanoparticles were designed to overcome the shortcoming of the uneven membrane size in the living organism. By exploiting the advantage of functional elements on human platelet membranes, this research group has developed cell membrane-based nanoparticles capable of targeting tumor cells and immune escape
The researchers have picked the membrane of human embryonic kidney-239T cells highly expressing human ACE2 receptor (HEK-293T-hACE2) to prepare cell membrane-based nanoparticles after analyzing the ACE2 content in five different human cells.
In short, HEK-293T-hACE2 cells were processed by repeated freezing and thawing in order to separate the membrane, which was broken by sonication, and subsequently applied to fabricate cell membrane-based nanoparticles using a classical extrusion method.
Moreover, to appraise the bioactivity of HEK-293T-hACE2 nanoparticles, the researchers have initially immobilized biotinylated SARS-CoV-2 S1-RBD on Sartorius streptavidin biosensors and then analyzed the recruitment of cell membrane-based nanoparticles by biolayer interferometry.
The antiviral activity and mechanism of action of this nanomaterial were explored in depth with the use of pseudovirus neutralization assay, spike adhesion experiment, as well as proteomics analysis. Finally, the toxicity of HEK-293T-hACE2 nanoparticles has been evaluated by a mouse experiment.
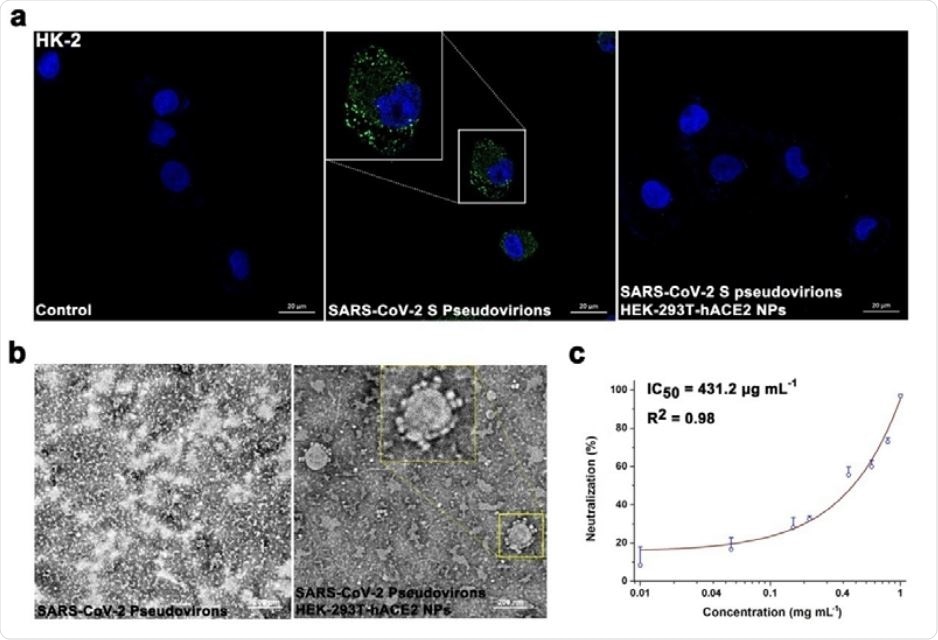
Efficient restriction of SARS-CoV-2 adherence
The content of ACE2 in HEK-293T-hACE2 nanoparticles (as determined by the enzyme-linked immunosorbent assay or ELISA) was 265.1 ng/mg, which was 3.2-fold higher than that in HEK-293T nanoparticles.
As a result of competitive inhibition, HEK-293T-hACE2 nanoparticles bound to SARS-CoV-2 S1 and subsequently blocked the viral ligand adhering to human renal tubular epithelial cells in a dose-dependent manner. The effect of SARS-CoV-2 S1 on cellular metabolism was suppressed by HEK-293T-hACE2 nanoparticles as well.
“Interestingly, SARS-CoV-2 S1 can translocate to the cytoplasm and affect the cell metabolism, which is also inhibited by HEK-293T-hACE2 nanoparticles”, study authors further explain. “This biocompatible membrane nanomaterial is sufficient to block the adherence of SARS-CoV-2 D614G-S1 mutant to sensitive cells”, they add.
As a result of the spike recruitment, HEK-293T-hACE2 nanoparticles adsorbed SARS-CoV-2 S pseudovirions on the material surface. They successfully blocked viral entry into the cytoplasm – enabling a rather inspiring type of host cell protection from the viral infection.
Promising therapeutic candidate
“Our study demonstrates an efficient nano-antagonist that can be easily prepared in general laboratories against SARS-CoV-2, which is a feasible solution to resolve the shortage of effective measures to treat COVID-19”, study authors summarize their main findings.
It was also shown that the overexpression of membrane receptors does not affect the biocompatibility of human cell membrane-based nanoparticles, while the demonstrated lack of toxicity in experimental animals laid a solid foundation for the application of HEK-293T-hACE2 nanoparticles as an antagonist to SARS-CoV-2.
And indeed, since there is still a lack of effective measures to treat COVID-19, this type of biocompatible and easy-to-achieve nano-antagonist may be a rather convenient therapeutic candidate for curbing the dire effect of SARS-CoV-2 spread. Naturally, further studies with a more practical, in vivo approach are needed.
Source: www.news-medical.net






















































