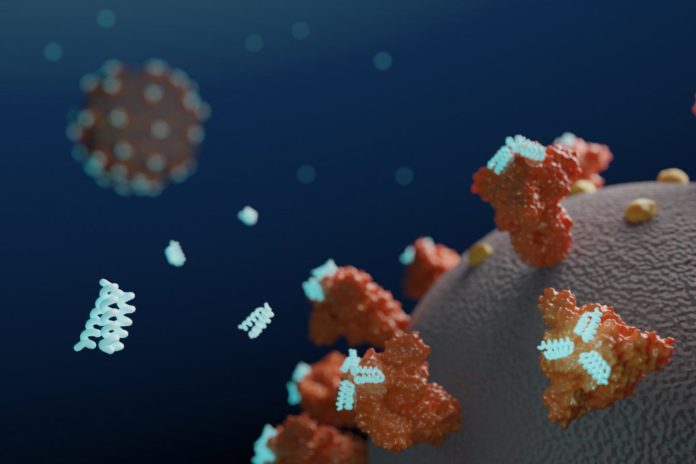
Researchers at the University of Washington (UW) School of Medicine Institute for Protein Design have used computers to design small proteins, or minibinders, that bind tightly to the SARS-CoV-2 Spike protein and prevent it from infecting cells. Their work has resulted in the development of a lead antiviral candidate, LCB1, which is currently undergoing evaluation in rodents, and which appears to rival the most effective known SARS-CoV-2 neutralizing antibodies in protecting against the virus.
“Although extensive clinical testing is still needed, we believe the best of these computer-generated antivirals are quite promising,” said Longxing Cao, PhD, a postdoctoral scholar at the Institute for Protein Design, and first author of the team’s paper, which is published in Science. “They appear to block SARS-CoV-2 infection at least as well as monoclonal antibodies, but are much easier to produce and far more stable, potentially eliminating the need for refrigeration.” Cao et al. outline their developments in a report titled, “De novo design of picomolar SARS-CoV-2 miniprotein inhibitors.”
Coronaviruses are studded with Spike proteins, which latch onto the ACE2 receptor on human cells as part of the mechanism for gaining entry and infecting these host cells. The development of drugs that interfere with this entry mechanism could enable the treatment of or even prevention of infection.
SARS-CoV-2 infection generally starts in the nasal cavity, where the virus replicates for a few days before spreading to the lower respiratory tract, the authors explained. “Delivery of a high concentration of viral inhibitor into the nose and into the respiratory system generally might therefore provide prophylactic protection and/or therapeutic benefit for treatment of early infection,” they wrote. This could be particularly useful for healthcare workers and others who come into frequent contact with infected individuals, they further suggested.
Although a number of monoclonal antibodies (mAbs) are in development as systemic treatments for COVID-19, such proteins aren’t ideal for intranasal delivery, as antibodies are large molecules, they are often not hugely stable, and they display low binding site density, effectively just two per antibody. “… antibody-dependent disease enhancement is also a potential issue,” the team noted. The ability to generate high-affinity Spike protein binders that block viral interaction with ACE2, but which are highly stable, and small—“to maximize the density of inhibitory domains”—could have multiple advantages over antibodies for direct delivery into the respiratory system. Such molecules could feasibly be delivered through intranasal administration, nebulization or dry powder aerosol.
The Protein Design Institute team set out to design high-affinity protein minibinders to the SARS-CoV-2 Spike receptor binding domain (RBD), which would compete with ACE2 binding. They did this via two approaches. For the first, they incorporated the alpha-helix segment from ACE2, which makes the majority of interactions with the RBD, into small protein scaffolds, which were designed to make additional interactions with the RBD as a means of generating higher affinity. Secondly, the scientists designed binders completely from scratch, without relying on known RBD-binding interactions. “An advantage of the second approach is that the range of possibilities for design is much larger, and so potentially a greater diversity of high-affinity binding modes can be identified,” they stated.
Scientists from the University of Washington School of Medicine in Seattle and Washington University School of Medicine in St. Louis collaborated on the work. Beginning in January, more than two million candidate Spike-binding proteins were designed on the computer. Over 118,000 were then produced and tested in the lab. Their tests suggested that the second, design from scratch, method produced the most potent antivirals, including the lead candidate, LCB1, which the researchers say is roughly six times more potent on a per mass basis than the most effective monoclonal antibodies reported thus far.
To confirm that the new antiviral proteins attached to the coronavirus Spike protein as intended, the team used cryo-electron microscopy to collect snapshots of the two molecules interacting. These experiments were performed by researchers in the laboratories of David Veesler, PhD, assistant professor of biochemistry at the UW School of Medicine, and Michael S. Diamond, PhD, the Herbert S. Gasser Professor in the Division of Infectious Diseases at Washington University School of Medicine in St. Louis.
“The minibinders designed in this work have potential advantages over antibodies as potential therapeutics,” the scientists reported in the Science paper. “Together, they span a range of binding modes, and in combination viral mutational escape would be quite unlikely.” And at a size that is 20-fold smaller than a full antibody molecule, an equal mass of the miniproteins could offer have 20-fold more potential neutralizing sites, “… increasing the potential efficacy of a locally administered drug,” they suggested. “The small size and high stability should also make them amenable to formulation in a gel for nasal application, and to direct delivery into the respiratory system by nebulization or as a dry powder.” The researchers further commented that going forwards, they will be looking at different routes of delivery, as they work to develop the high potency neutralizing proteins into SARS-CoV-2 therapeutics and prophylactics.
“Our success in designing high-affinity antiviral proteins from scratch is further proof that computational protein design can be used to create promising drug candidates,” said senior author and Howard Hughes Medical Institute Investigator David Baker, PhD, professor of biochemistry at the UW School of Medicine and head of the Institute for Protein Design.
“The hyperstable minibinders provide promising starting points for new SARS-CoV-2 therapeutics,” the antiviral research team wrote in their study pre-print, “and illustrate the power of computational protein design for rapidly generating potential therapeutic candidates against pandemic threats.”
source: www.genengnews.com






















































