Study shows that nanoparticles serve as a good tumor deoxygenation agent
One target therapy in cancer research is to suffocate the tumor. Cells need oxygen to survive so researchers have focused on methods for cutting off the blood supply to the tumor. Very little research has involved the direct removal of oxygen within the tumor.
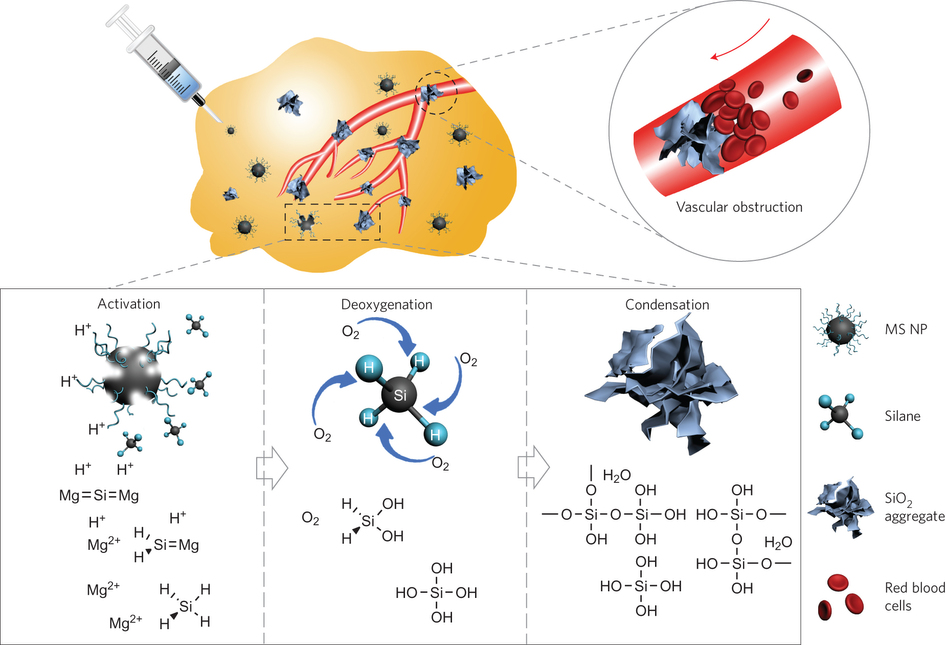
To this end, a group of researchers from the Shanghai Institute of Ceramics, Chinese Academy of Sciences and the East China Normal University have developed a deoxygenation agent using polyvinyl pyrrolidone modified Mg2Si nanoparticles. This agent is pH sensitive, efficiently consumes oxygen, and one of the products of oxygen consumption also forms aggregates that could potentially block blood vessels. Preliminary mouse studies show tumor hypoxia and good biocompatibility. Their work appears in Nature Nanotechnology.
There are several important qualities for a good tumor-starving agent. For one, the agent must be biocompatible which negates the use of heavy metals for oxygen absorption. Additionally, the agent must be efficient at deoxygenation and serve as a long-term oxygen scavenger including preventing re-oxygenation of deoxygenated tumors through undamaged blood vessels. And, as always, any cancer treatment needs to target tumors without damaging healthy tissue, and the agent should be easily injectable by syringe.
In the current research, Zhang et al. developed polyvinyl pyrrolidine (PVP)-modified Mg2Si nanoparticles that have several of the qualities for a good tumor-starving agent. Importantly, the main components, magnesium, silicon dioxide, and water are biocompatible. Additionally, the reaction mechanism forms a highly reactive O2 scavenger, SiH4, which serves to make these nanoparticles highly efficient at scavenging oxygen.
In order to make injectable nanoparticles, Zhang et al. developed a self-propagating high-temperature synthesis in an oxygen-argon atmosphere. This allows the nanoparticles to remain dispersed in the liquid, rather than form clusters, so that they are injectable into tissue. This synthesis takes advantage of the formation of MgO by-product that halts the continual formation of Mg2Si aggregates.
source : phys.org