
Buzzed flies reveal an indirect path to intoxication. [Scripps Research]
At the molecular level, the pathway to drunkenness isn’t a straight line. It swerves toward an intermediate step, one that was overlooked in earlier studies of alcohol anesthesia. According to a new study of alcohol anesthesia from the Scripps Research Institute, ethanol intake causes a “buzzed” sensation when a membrane-bound enzyme produces lipid-alcohol metabolites that bind to and regulate downstream activities in nerve cells. When the metabolite known as phosphatidylethanol (PEtOH) builds up, it causes nerve cells to fire more easily, inducing alcohol’s familiar buzz.
To uncover this crooked path, a Scripps team led by Scott B. Hansen, Ph.D., plied flies with alcohol and traced the molecular-level results. Flies, like people, can get drunk on alcohol. And the fly, Dr. Hansen pointed out, is an especially useful model of drunkenness because its genome is smaller than that of other animals and is easily manipulated.
Detailed findings about the newly discovered pathway behind alcohol sensitivity appeared in the Journal of Molecular Biology, in an article titled, “A Molecular Target for an Alcohol Chain-Length Cutoff.” These findings, Dr. Hansen suggested, could enable development of an antidote to intoxication, or even hangover.
“In animals, the enzyme phospholipase D (PLD) has been shown to generate alcohol metabolites with a cutoff, but no phenotype has been shown connecting PLD to an anesthetic effect,” the article’s authors wrote. “Here we show loss of PLD blocks ethanol-mediated hyperactivity in Drosophila melanogaster (fruit fly), demonstrating that PLD mediates behavioral responses to alcohol in vivo.”
Previous studies had shown that n-alcohols induce anesthesia up to a specific chain length and then lose potency—an observation known as the “chain-length cutoff effect.” This cutoff effect has been thought to be mediated by alcohol binding sites on proteins such as ion channels. It has been unclear, however, where these sites are located for long-chain alcohols. Also blurred were the mechanisms that could explain how these sites mediate the cutoff effect.
In the current study, a crucial move was to knock out the gene for the enzyme that makes the PEtOH metabolite. Doing so, the Scripp team reported, eliminates the metabolite’s signaling function and prevents hyperactivity, the fly behavior associated with drunkenness.
“The metabolite PEtOH directly competes for the endogenous PLD product phosphatidic acid at lipid-binding sites within potassium channels [e.g., TWIK-related K+ channel type 1 (K2P2.1, TREK-1)],” the article’s authors explained. “This gives rise to a PLD-dependent cutoff in TREK-1.”
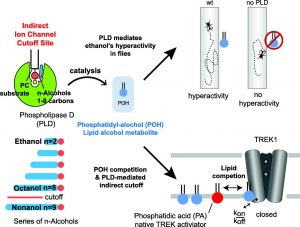
Graphical abstract from Scripps Research Institute study on alcohol chain-length cutoff. [Journal of Molecular Biology]
This is the first time this pathway has been identified as a determinant of alcohol sensitivity, Dr. Hansen emphasized. It remains to be seen whether the metabolite is involved in the full sedation experienced by the flies after the initial buzz and how this pathway may play a role in the hangover that many people experience later on. These questions are being addressed in additional research being conducted by Dr. Hansen’s team.
“The fatty alcohol is known to linger in the brain for more than 16 hours making it a likely target,” Dr. Hansen said. “Also, understanding this pathway could give insight as to why people use alcohol for pain management.”
“[Our findings have] definitely led to some different ways of thinking about alcohol intoxication at the molecular level,” Dr. Hansen asserted. “Most scientists thought alcohol had a direct effect. Blocking the enzyme in flies shows that’s not likely true.”
Source: www.genengnews.com






















































