Gates/Bezos-funded charity champions research into methods for early detection of Alzheimer’s disease
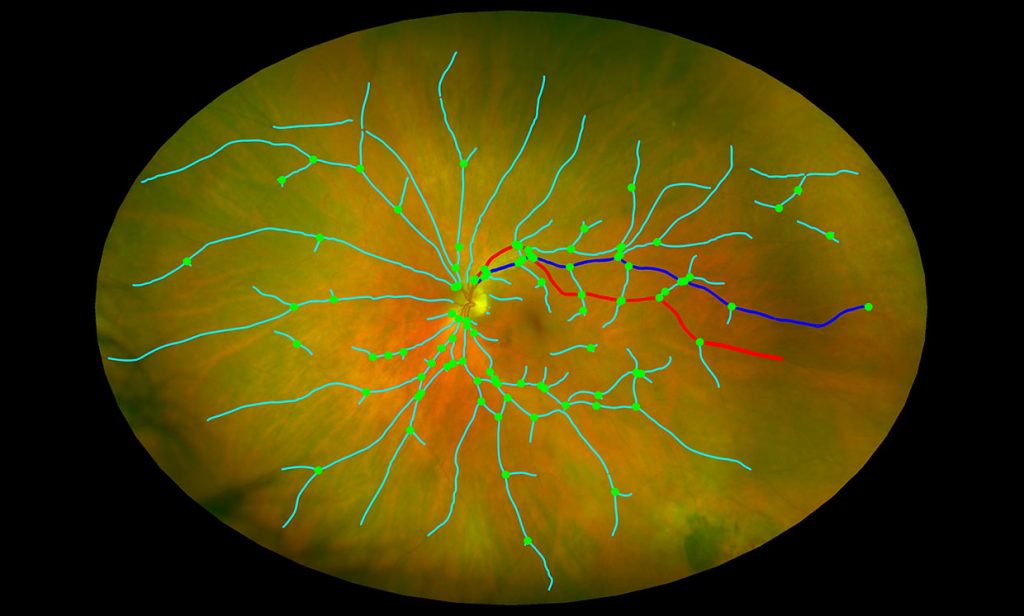
An image of the retina from an ultra-widefield scanning laser ophthalmoscope tracks the branching patterns of small blood vessels.
The Alzheimer’s Drug Discovery Foundation (ADDF) just announced the first award recipients of their $50 million Diagnostics Accelerator research program, an initiative funded by Bill Gates, Jeff and MacKenzie Bezos, and former Estée Lauder CEO Leonard Lauder, among others.
The four recipients, chosen from a pool of 300 applicants across 30 countries, are developing reliable, cost-effective ways to diagnose Alzheimer’s disease, including one that will use machine learning to detect early signs of concern through an eye scan.
“Unlike heart disease and cancer, we lack simple and cost-effective diagnostic tools and biomarkers that are critical to finding ways to prevent and treat Alzheimer’s disease,” said ADDF chief scientific officer Howard Fillit in a press release. “Once we have them, we will better understand how Alzheimer’s progresses and make clinical drug trials more efficient and rigorous.”
Current tests to diagnose Alzheimer’s disease (AD) are expensive, often invasive, and catch the disease only after symptoms have emerged. These tests include cognitive evaluations, such as memorization exercises; neuroimaging via costly PET and MRI scans; and measuring levels of Alzheimer’s associated proteins, amyloid beta and tau, in the cerebral spinal fluid.
In contrast to those existing options, the ADDF winners want to make tests that are less expensive, portable, and detect AD far earlier in life.
At the University of Edinburgh in Scotland, Tom MacGillivray received $488,997 to develop a comprehensive eye scan system analyzing multiple biomarkers in the eye to detect brain degeneration. “We can look inside the human body through the natural window of the retina,” he told IEEE Spectrum.
The comprehensive scanning procedure, including image analysis software with machine learning components, would analyze images from eye-scanning equipment that’s already available in optician offices (and is far less expensive than MRI or PET machines). Working with Sharon Fekrat at Duke University, MacGillivray will measure changes in small blood vessels at the back of the eye and changes in layers of nerve tissue which have been associated with AD.
“Some of these changes in the eye might predate some of the serious cognitive decline symptoms that present later on in the disease,” says MacGillivray. If such changes can be detected early, then it might be possible to intervene early and test out new preventative drugs, he adds.
Ideally, the resulting system could be adapted into a cloud-based system so doctors could upload images taken at eye clinics for analysis, says MacGillivray. ADDF has provided 18 months of funding, in which time the team will gather enough data to “tell us if this has a future or not,” he adds.
Peter van Wijngaarden (left) and Xavier Hadoux are developing a specialized eye imaging method to detect amyloid proteins in the retina
At the Centre for Eye Research Australia and the University of Melbourne, Peter van Wijngaarden received $420,321 from the ADDF to develop an eye scan to detect amyloid beta in the retina using a low-cost prototype camera.
“Instead of imaging the retina with a single white light flash, as is done in conventional retinal photography, we image the retina sequentially with many different wavelengths of light,” says van Wijngaarden. The technique, known as hyperspectral imaging, produces detailed information about the structure of the retina, which is then processed by software to distinguish between individuals with high levels of amyloid beta and those without.
The team plans to use the ADDF funding to participate in an early-screening study of people at high risk of developing Alzheimer’s, in which they will compare their retinal results to other biomarkers.
The final two winners seek to measure markers of Alzheimer’s in the blood: Saliha Moussaoui at Amoneta Diagnostics SAS in France will receive up to $2 million for a test to measure two types of RNA associated with cognitive decline; and Kaj Blennow at the University of Gothenburg in Sweden was awarded $500,000 for a blood test for tau protein.
While the ADDF funding gives these four technologies a boost, they’re not the only ones seeking to diagnose and monitor AD more effectively: Sacramento-based Neurovision is also seeking to detect amyloid deposits in the eye as an early diagnostic, while San Francisco start-up NeruaMetrix wants to measure brain health by tracking typing habits.
source: www.spectrum.ieee.org





















































